Management Strategies
Figure 1: Disease Pathogenesis and Potential Treatments for COVID-19 Based on Disease Severity10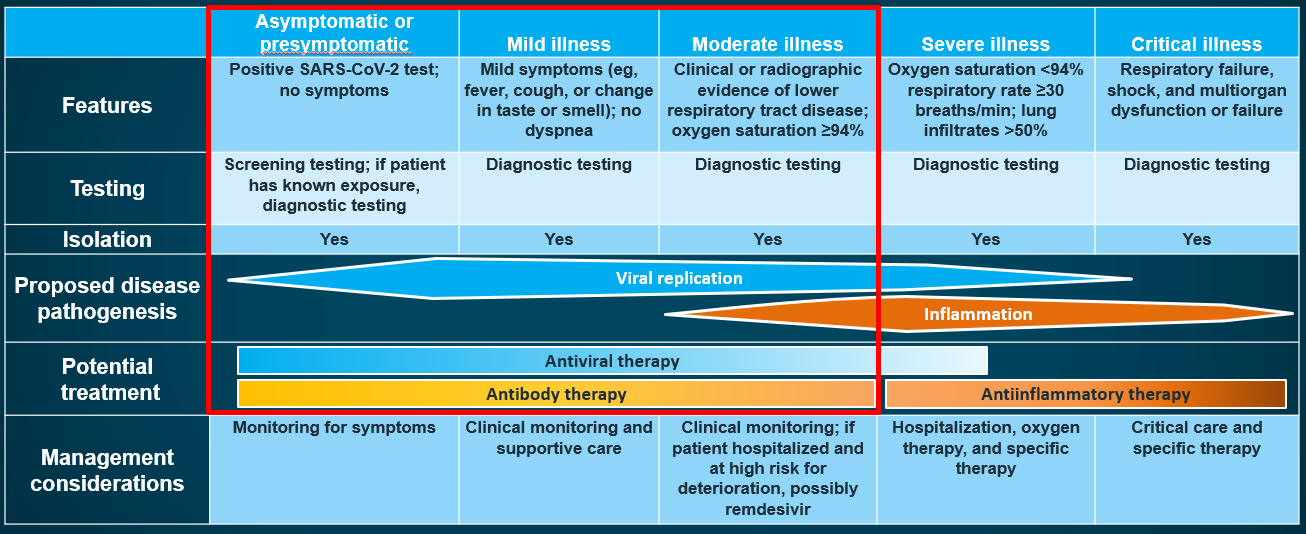
Mild illness. Patients with mild illness may present with any signs or symptoms of COVID-19 (eg, fever, cough, muscle pain, sore throat, malaise, headache, nausea, vomiting, diarrhea, loss of taste and smell); however, they do not exhibit shortness of breath, dyspnea on exertion, or abnormal chest imaging results.1 Most cases of mild illness can be managed through telemedicine or in the ambulatory setting. No imaging or laboratory testing is routinely indicated in otherwise healthy patients with mild COVID-19.1 Any patient with risk factors for severe disease should be monitored closely because the clinical course of the disease may progress rapidly in some patients (about 1 week after illness onset).1,2,11,12 Risk factors for severe COVID-19 include, but are not limited to, cardiovascular disease, chronic lung disease, sickle cell disease, diabetes, cancer, obesity, and chronic kidney disease, as well as being 65 years or older, pregnant, a smoker, or a recipient of transplant or immunosuppressive therapy.1 The anti-SARS-CoV-2 monoclonal antibody bebtelovimab is effective against current circulating Omicron subvariants and is available under an EUA for outpatients with mild COVID-19 who are at high risk for progressing to severe disease and/or hospitalization. Bebtelovimab should be administered as soon as possible and within 7 days of symptom onset. In clinical trials, monoclonal antibodies have been shown to reduce viral load and decrease the number of patients requiring medical care and hospitalization.4,7 The antiviral therapies nirmatrelvir/ritonavir and molnupiravir are also authorized under EUAs for the treatment of mild COVID-19 in high-risk patients. These oral therapies should be administered as soon as possible and within 5 days of symptom onset.8,9 Remdesivir may also be considered for patients with mild disease at high-risk for progression to severe COVID-19, including hospitalization and death. It should be administered as soon as possible after diagnosis of symptomatic COVID-19 and within 7 days of symptom onset.4
Moderate illness. Moderately ill patients have clinical or imaging evidence of lower respiratory disease, with SpO2 ≥94% on room air at sea level.1 These patients should be monitored closely, given the risk of rapid disease progression that may require hospitalization. The initial evaluation should include chest imaging by x-ray, ultrasound, or computed tomography (CT). An electrocardiogram should be performed if indicated.1 Complete blood count (CBC) with differentials and a metabolic profile, including liver and renal function tests, should be performed. Measurements of inflammatory markers, such as C-reactive protein (CRP), D-dimer, and ferritin, may have prognostic value.1 Several therapies are available for the management of moderate COVID-19, including bebtelovimab, nirmatrelvir/ritonavir, molnupiravir, and remdesivir. See the previous section on Mild Illness for additional information on using these therapies.4,7-9
If you have any further questions surrounding COVID-19 hospitalized and non-hospitalized patient management strategies, contact the Med Learning Group today.
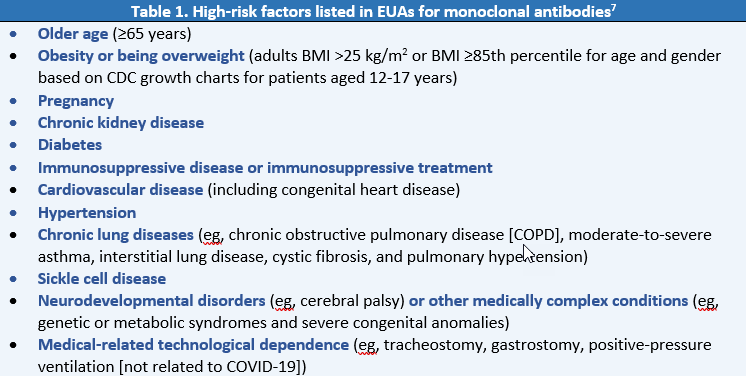
Authorization of monoclonal antibodies is not limited to medical conditions listed in the EUAs. A list of additional high-risk factors can be found on the CDC website: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html
Severe illness. Severely ill patients have SpO2 <94% on room air at sea level, respiratory rate >30/min, PaO2/FiO2 <300 mm Hg, or lung infiltrates >50%.1 Oxygen therapy should be initiated immediately, using a nasal cannula or high-flow oxygen. These patients should be admitted to a healthcare facility.1,2 Evaluation should include chest imaging, CBC, and a metabolic panel. Measurements of inflammatory markers may have prognostic value.1 Available treatment options for hospitalized patients with severe COVID-19 who require supplemental oxygen include remdesivir, dexamethasone, baricitinib, or tocilizumab.2,4-6 Given the potential of a hypercoagulable state and the high incidence of thrombosis in hospitalized patients with COVID-19, clinicians should have a high clinical suspicion for thrombotic events. All hospitalized patients with COVID-19 should receive prophylactic dose anticoagulation.13-15 Choice of therapy should depend on factors such as oxygen needs and presence of systemic inflammation. For patients requiring supplemental oxygen, dexamethasone and/or remdesivir are recommended therapies. For patients on dexamethasone with rapidly increasing oxygen needs and systemic inflammation, baricitinib or tocilizumab may be added. Patients requiring oxygen through a high-flow device or noninvasive ventilation should receive dexamethasone with or without remdesivir. Baricitinib or tocilizumab should be added if oxygen needs increase rapidly and systemic inflammation is present. For patients on mechanical ventilation or ECMO, dexamethasone is recommended. Patients within 24 hours of admission to the ICU should receive dexamethasone plus tocilizumab.15
Critical illness. Critically ill patients may have acute respiratory distress syndrome (ARDS), virus-induced distributive septic shock, cardiac dysfunction, cytokine storm from elevations in inflammatory cytokines, multiorgan dysfunction, and/or exacerbation of underlying comorbidities. In addition to pulmonary disease, patients with critical COVID-19 may also develop cardiac, hepatic, renal, central nervous system, or thrombotic disease.1 Care of critically ill patients with COVID-19 requires treating both the medical condition that initially resulted in the critical illness (ie, COVID-19 from SARS-CoV-2 infection) plus other comorbidities and nosocomial complications.1 Available treatment options for critically ill patients with COVID-19 include dexamethasone, remdesivir, baricitinib, or tocilizumab.2,4-6 All critically ill patients with COVID-19 should receive prophylactic dose anticoagulation.13-15
Pharmacologic Management of Patients Hospitalized with COVID-19
Remdesivir. Remdesivir is an inhibitor of SARS-CoV-2 nucleotide analog RNA polymerase.4 It is approved by the FDA for the treatment of COVID-19 in those requiring hospitalization, based on data from clinical trials showing that it can reduce time to recovery in hospitalized patients with COVID-19.4,16-18 It is also approved for the treatment of patients with mild-to-moderate COVID-19 who are at high risk for progression to severe disease, including hospitalization and death, if administered as soon as possible and within 7 days of symptom onset.4
In the ACTT-1 trial, remdesivir was associated with a shorter median time to recovery compared to placebo (10 days vs 15 days; recovery rate ratio, 1.29; P< .001) in patients with mild-to-severe disease.16 Another trial in hospitalized patients with severe disease found that subjects receiving a 5-day course of remdesivir had similar clinical status at day 14 as those receiving a 10-day course (odds ratio, 0.75; 95% CI, 0.51-1.12).17 In non-hospitalized patients with mild-to-moderate COVID-19 who were at high risk for progression to severe disease, remdesivir reduced the proportion of patients with COVID-19-related hospitalization or all-cause mortality through day 28 compared to placebo (0.7% vs 5.3%; hazard ratio, 0.13; 95% CI, 0.03-0.59; P= .008).18
Remdesivir is given by IV injection and should only be administered in a hospital or in a healthcare setting capable of providing acute care comparable to inpatient hospital care.4 The most common adverse reactions observed with treatment with remdesivir are nausea and elevated levels of aspartate aminotransferase and alanine aminotransferase.4
Dexamethasone. RECOVERY and other clinical trials showed that treatment with dexamethasone improves survival among hospitalized patients with severe COVID-19 who require supplemental oxygen, including critically ill patients on ventilatory support.19-21 Among critically ill patients, the odds of mortality at day 28 was 34% less among patients treated with corticosteroids compared to those not treated with corticosteroids (odds ratio, 0.66). Among those with severe illness, 28-day mortality was 17% lower in the group that received dexamethasone. In addition, patients with severe or critical disease were more likely to be discharged from the hospital at 28 days. However, there was no evidence of benefit and trend toward harm with dexamethasone in those without hypoxia not receiving supplemental oxygen (rate ratio, 1.19).
Baricitinib. Baricitinib is an oral Janus kinase (JAK) inhibitor with anti-inflammatory as well as potential antiviral activity.22 The FDA has approved the use of baricitinib for the treatment of hospitalized patients aged 2 years or older who require supplemental oxygen, invasive mechanical ventilation, or extracorporeal membrane oxygenation (ECMO).5
The ACTT-2 trial showed that baricitinib plus remdesivir is superior to remdesivir alone in reducing recovery time among hospitalized patients with COVID-19, especially those receiving high-flow oxygen or noninvasive ventilation. Patients receiving baricitinib plus remdesivir were also more likely to have a better clinical status at day 15 compared with patients assigned to placebo plus remdesivir.5,22 The phase 3 COV-BARRIER study found a significant reduction in the proportion of hospitalized patients who died by day 28 with baricitinib versus placebo (8% vs 13%, respectively; hazard ratio = 0.57 (95% CI, 0.41-0.78)).23 Known adverse effects of baricitinib include serious venous thrombosis, such as pulmonary embolism, and serious infections.5
Tocilizumab. Tocilizumab is an IL-6 inhibitor that blocks soluble and membrane-bound receptors for IL-6, a pro-inflammatory cytokine. Excessive systemic inflammation and elevated IL-6 levels resulting from dysregulated host immune responses are associated with adverse clinical outcomes in patients hospitalized with COVID-19.24 The FDA issued an EUA for tocilizumab for the treatment of COVID-19 in hospitalized adults and pediatric patients aged 2 years and older who are receiving systemic corticosteroids and require supplemental oxygen, non-invasive or invasive mechanical ventilation, or ECMO.6
This authorization is based on the results of the RECOVERY and REMAP-CAP trials. In the RECOVERY trial, tocilizumab improved survival and other clinical outcomes in patients with clinical evidence of progressive COVID-19, defined as <92% oxygen saturation on room air or receiving oxygen and C-reactive protein ≥75 mg/L. Compared to standard of care alone, patients receiving tocilizumab in addition to standard of care were less likely to die within 28 days (31% vs 35%; rate ratio, 0.85; P= .0028) and were more likely to be discharged from the hospital within 28 days (57% vs 50%; rate ratio, 1.22; P< .0001).25 In the REMAP-CAP trial, adult patients with COVID-19 were randomized to tocilizumab, sarilumab, or placebo within 24 hours after starting organ support in the intensive care unit (ICU). The median number of organ support-free days was 10 with tocilizumab, 11 with sarilumab, and 0 in the control group. An analysis of 90-day survival showed improved survival in the pooled IL-6 receptor antagonist groups when compared to the control group (hazard ratio, 1.61; 95% CI, 1.25-2.08).26
References
- National Institutes of Health (NIH). Clinical spectrum of SARS-CoV-2 infection. In: Coronavirus disease 2019 (COVID-19) treatment guidelines. https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/
- National Institutes of Health (NIH). COVID-19 Treatment Guidelines. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Accessed June 5, 2022. https://files.covid19treatmentguidelines.nih.gov/guidelines/covid19treatmentguidelines.pdf
- Infectious Diseases Society of America (IDSA). Guidelines on the treatment and management of patients with COVID-19. https://www.idsociety.org/globalassets/idsa/practice-guidelines/covid-19/treatment/tx-and-mgmt-v5.4.1-1.pdf
- VEKLURY® (remdesivir). Prescribing information. Gilead Sciences, Inc; 2022. https://www.gilead.com/-/media/files/pdfs/medicines/covid-19/veklury/veklury_pi.pdf
- OLUMIAN (baricitinib). Prescribing information. Eli Lilly and Company; 2022. https://pi.lilly.com/us/olumiant-uspi.pdf.
- US Food and Drug Administration (FDA). Emergency use authorization (EUA) for ACTEMRA® (tocilizumab). https://www.fda.gov/media/150321/download
- US Food and Drug Administration (FDA). Emergency use authorization (EUA) for bebtelovimab. https://www.fda.gov/media/156152/download
- US Food and Drug Administration (FDA). Emergency use authorization (EUA) for PAXLOVID™ (nirmatrelvir/ritonavir). https://www.fda.gov/media/155050/download
- US Food and Drug Administration (FDA). Emergency use authorization (EUA) for LAGEVRIO™ (molnupiravir). https://www.fda.gov/media/155054/download
- Gandhi RT, Lynch JB, Del Rio C. Mild or moderate COVID-19. N Engl J Med. 2020;383:1757-1766. doi:10.1056/NEJMcp2009249
- Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708-1720. doi:10.1056/NEJMoa2002032
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. doi:10.1016/S0140-6736(20)30183-5
- Infectious Diseases Society of America (IDSA). COVID-19 real-time learning network. Thrombosis. Last reviewed January 20, 2022. https://www.idsociety.org/covid-19-real-time-learning-network/disease-manifestations-complications/thrombosis/
- National Institutes of Health (NIH). Coronavirus disease 2019 (COVID-19) treatment guidelines. Antithrombotic therapy in patients with COVID-19. Last Updated May 31, 2022. https://www.covid19treatmentguidelines.nih.gov/adjunctive-therapy/antithrombotic-therapy/
- National Institutes of Health (NIH). COVID-19 Treatment Guidelines. Clinical Management Summary. Last Updated April 8, 2022 https://www.covid19treatmentguidelines.nih.gov/management/clinical-management/clinical-management-summary/
- Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19-final report. N Engl J Med. 2020;383:1813-1826. doi:10.1056/NEJMoa2007764
- Goldman JD, Lye DCB, Hui DS, et al. Remdesivir for 5 or 10 days in patients with severe Covid-19. N Engl J Med. 2020;383:1827-1837. doi:10.1056/NEJMoa2015301
- Gottlieb RL, Vaca CE, Paredes R, et al. Early remdesivir to prevent progression to severe COVID-19 in outpatients. N Engl J Med. 2022;386:305-315. doi:10.1056/NEJMoa2116846
- RECOVERY Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693-704. doi:10.1056/NEJMoa2021436
- Tomazini BM, Maia IS, Cavalcanti AB, et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: The CoDEX randomized clinical trial. JAMA. 2020;324:1307-1316. doi:10.1001/jama.2020.17021
- WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group, Sterne JAC, Murthy S, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: A meta-analysis. JAMA. 2020;324:1330-1341. doi:10.1001/jama.2020.17023
- Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus remdesivir for hospitalized adults with Covid-19. N Engl J Med. 2021;384:795-807. doi:10.1056/NEJMoa2031994
- Marconi VC, Ramanan AV, de Bono S, et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): A randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. Lancet Respir Med. 2021;9(12):1407-1418. doi:10.1016/S2213-2600(21)00331-3
- WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group, Shankar-Hari M, Vale CL, et al. Association between administration of IL-6 antagonists and mortality among patients hospitalized with COVID-19: A meta-analysis. JAMA. 2021;326:499-518. doi:10.1001/jama.2021.11330
- RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet. 2021;397:1637-1645. doi:10.1016/S0140-6736(21)00676-0
- REMAP-CAP Investigators, Gordon AC, Mouncey PR, et al. Interleukin-6 receptor antagonists in critically ill patients with COVID-19. N Engl J Med. 2021;384:1491-1502. doi:10.1056/NEJMoa2100433





