Prevention and Diagnosis
Clinical Presentation
The estimated incubation period for COVID-19 is up to 14 days from the time of exposure, with a median incubation period of approximately 4 to 5 days.1 Patients with COVID-19 often have clinical manifestations of fever (85–89%), cough (68–86%), and shortness of breath (19–80%). In a study of 1099 confirmed cases hospitalized with COVID-19, fever was present in 43.8% of patients at admission but ultimately developed in 88.7% of patients during hospitalization.2,3
Less common symptoms are nausea (24%), muscle ache (11–34%), confusion (9%), headache (8–16%), sore throat (5–18%), nasal congestion/rhinorrhea (4–16%), chest pain (2–15%), and diarrhea (2–27%). Other reported symptoms have included anosmia, dysgeusia, sputum production, dizziness, abdominal pain, anorexia, and vomiting. 2–4
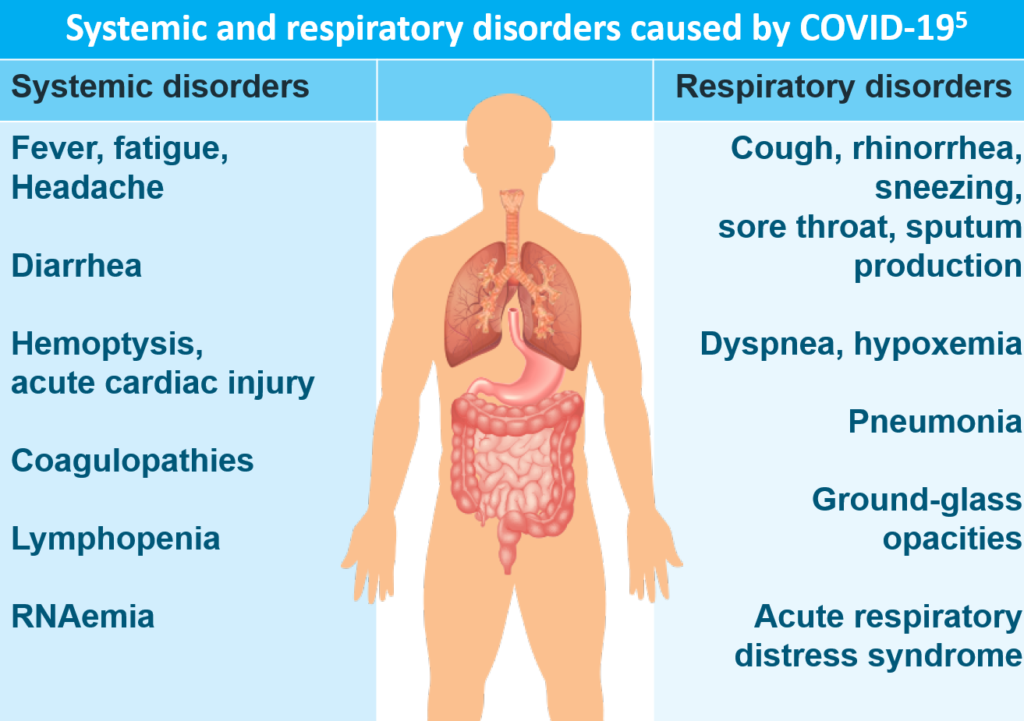
Clinical Course
The clinical spectrum of COVID-19 ranges from asymptomatic to respiratory failure to multiorgan and systemic manifestations. A large study of 44,672 confirmed COVID-19 cases identified by the Chinese Centers for Disease Control and Prevention early in the pandemic found that6:
- 81% of the cases were mild (ie, no pneumonia and mild pneumonia)
- 14% of the cases were severe (ie, dyspnea, respiratory frequency ≥30/min, blood-oxygen saturation ≤93%, partial pressure of arterial oxygen to fraction of inspired oxygen ratio <300 mm Hg, and/or lung infiltrates >50% within 24 to 48 hours)
- 5% of the cases were critical (ie, respiratory failure, septic shock, and/or multiple-organ dysfunction or failure)
The overall case-fatality rate (CFR) identified in this study was 2.3%. No deaths were reported among mild and severe cases, but the CFR was 49% among critical cases.6
The median time from symptom onset to the development of pneumonia is 5 days, and the median time from symptom onset to severe hypoxemia and admission to the intensive care unit (ICU) is 7–12 days. Acute hypoxemic respiratory failure from acute respiratory distress syndrome (ARDS) is the most common complication, which is found in 60–70% of patients admitted to the ICU. Shock (30%), myocardial dysfunction (20–30%), and acute kidney injury (10–30%) also account for a substantial number of complications related to COVID-19.7
Risk Factors for Severe Illness
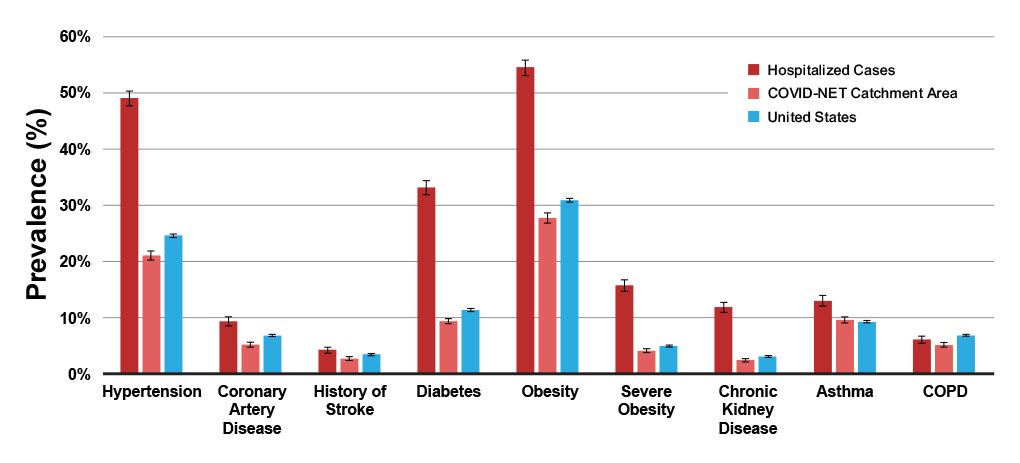
In a study of 44,672 confirmed COVID-19 cases in China, the CFR was elevated among those patients with preexisting comorbid conditions, including cardiovascular disease (10.5%), diabetes (7.3%), chronic respiratory disease (6.3%), hypertension (6.0%), and cancer (5.6%).6 A study of 1482 patients hospitalized with COVID-19 in the United States (US) found that 89.3% had one or more underlying conditions, the most common being hypertension (49.7%), obesity (48.3%), chronic lung disease (34.6%), diabetes mellitus (28.3%), and cardiovascular disease (27.8%).3
Figure 1. Prevalence of underlying medical conditions in adults with COVID-19-associated hospitalizations8
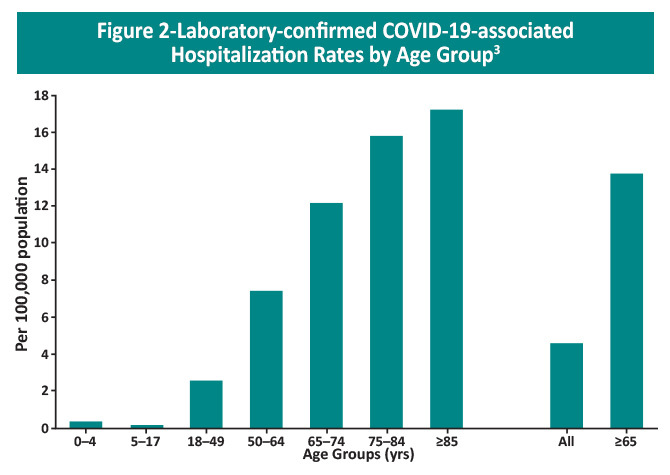
Age is another important factor impacting disease severity. Compared with the overall CFR of 2.3% in the study in China, the CFR was elevated in patients ≥80 years of age (14.8%) and in those aged 70–79 years (8.0%).6 A preliminary analysis of the mortality rate in the US between February 12 and March 16, 2020 found that fatality from COVID-19 ranged from 10% to 27% in persons aged ≥85, followed by 3% to 11% in persons aged 65–84 years, 1% to 3% among persons aged 55–64 years, and <1% in persons aged 20–54 years.9 It is important to note that risk increases steadily with increasing age and severe illness is possible in patients under 65 years of age.
The Centers for Disease Control and Prevention (CDC) have also identified other factors and medical conditions that increase the risk of severe illness from COVID-19 in all age groups. Long-standing systemic health and social inequalities place many groups at increased risk, particularly people from racial and ethnic minority groups and those with disabilities. Additional risk factors can be found in the table below. Vaccination is key to preventing severe illness in these individuals.10 Additionally, individuals with any of these high-risk factors are eligible to receive monoclonal antibody therapy as post-exposure prophylaxis or as early treatment for mild-to-moderate COVID-19 to prevent progression to severe illness.
| Table 2. Risk factors for progression to severe COVID-1910 | |
|
|
Laboratory Findings
A retrospective study of 150 patients in Wuhan, China identified several predictors of fatal outcome in COVID-19 cases, including older age, the presence of underlying diseases or secondary infections, and elevated inflammatory indicators in the blood. Laboratory results showed significant differences in white blood cell counts and platelets, cardiac troponin, myoglobin, C-reactive protein (CRP), and interleukin-6 (IL-6) between patients who recovered from COVID-19 and those who died from viral complications.11 A meta-analysis found the most frequent laboratory abnormalities with COVID-19 were lymphopenia (35–75% of cases), increased CRP levels (75–93% of cases), lactate dehydrogenase (LDH) (27–92% of cases), erythrocyte sedimentation rate (ESR) (up to 85% of cases), and D-dimer (36–43% of cases), as well as low concentrations of serum albumin (50–98% of cases) and hemoglobin (41–50%). Laboratory abnormalities that were predictive of an adverse outcome included lymphopenia, neutrophilia, and elevated levels of LDH, CRP, alanine aminotransferase (ALT), and aspartate aminotransferase (AST).12
COVID-19 patients with ARDS showed abundant interstitial mononuclear inflammatory infiltrate in the lungs, particularly lymphocytes, implying that immune hyperactivation may be at least partially responsible for the severity of COVID-19 in these patients.13 These laboratory features, combined with the fever and confusion commonly found in critically ill patients infected with COVID-19, suggest the presence of a cytokine storm syndrome (CSS) that results in ARDS and multiorgan failure.4,14,15 CSS is believed to be a consequence of an accentuated immune response to various triggers, including certain viral infections.4,15
Prevention
Vaccine clinical trial information. Since the beginning of the pandemic, vaccines against infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have been aggressively pursued.18 Standard vaccine platforms, as well as novel approaches (eg, mRNA vaccines), have been used to develop vaccine candidates. Currently, three vaccines have been approved or authorized for emergency use against the SARS-CoV-2 virus.
Two mRNA vaccines that encode the SARS-CoV-2 spike protein—Comirnaty® (BNT162b2) by Pfizer-BioNTech and mRNA-1273 by Moderna—are now available for the prevention of COVID-19.19,20 The Pfizer-BioNTech vaccine is approved for individuals 16 years of age or older and authorized for children aged 5-15 years. The Moderna vaccine is authorized for emergency use in individuals 18 years of age or older. Both vaccines are given intramuscularly in 2 doses (21 days apart for the Pfizer-BioNTech vaccine and 28 days for the Moderna vaccine). Available phase 3 trial data indicate that both vaccines have largely similar efficacy and safety profiles.21,22 Both vaccines show high efficacy (~95%) in preventing symptomatic COVID-19 infections. Vaccine recipients reported local (pain, swelling, etc) and systemic reactions (fatigue, headache, fever, chills, etc), which were mostly mild to moderate and resolved rapidly. Reactions were reported more often by younger vaccine recipients than older recipients and more commonly after the second dose than the first dose.
Protection against the virus may decrease over time, and booster shots are recommended. The Pfizer-BioNTech vaccine is authorized for a single booster shot in adults and children aged 5 years or greater, a second booster in individuals 50 years and older, and a second booster in individuals 12 years and older with certain kinds of immunocompromise. The Moderna vaccine is authorized as a single booster in all individuals 18 years and older and a second booster in individuals 50 years and older and adults who are moderately or severely immunocompromised.23
In addition to the two mRNA vaccines, an adenovirus vector vaccine—Ad26.CoV2.S by Johnson & Johnson/Janssen—is also authorized for emergency use for the prevention of COVID-19. Ad26.COV2.S is indicated for use in individuals 18 years of age or older and is administered as a single dose. This vaccine was found to be 85% effective in preventing severe disease and 66.3% effective against symptomatic, laboratory-confirmed COVID-19. A warning has been added to the vaccine’s EUA label following 15 identified cases of thrombosis-thrombocytopenia syndrome (TTS) in women aged 18 to 59 years. TTS is characterized by a type of blood clot called cerebral venous sinus thrombosis (CVST) in combination with low levels of blood platelets (thrombocytopenia). Reports indicate symptom onset between 6 and 15 days after vaccination.24,25
The CDC has issued and constantly updates its clinical guidance guidelines for healthcare professionals and pharmacists on COVID-19 vaccination, including information on contraindications and precautions, storage and handling, administration, management, reporting of adverse effects, and patient education for each specific vaccine.26
Post-exposure prophylaxis. Casirivimab plus imdevimab and bamlanivimab plus etesevimab were previously authorized for post-exposure prophylaxis in individuals 12 years and older who are at high risk for progression to severe COVID-19 and are not fully vaccinated or who are not expected to mount an adequate immune response to SARS-CoV-2 vaccination, such as those who are immunocompromised, AND
- Have been in close contact with an individual infected with SARS-CoV-2 (see close contact criteria per the CDC) OR
- Who are at high-risk for exposure to an individual with SARS-CoV-2 in an institutional setting27,28
However, these monoclonal antibodies are not effective against the Omicron variant and are not currently authorized for post-exposure prophylaxis against this variant.27,28
Pre-exposure prophylaxis. The monoclonal antibodies tixagevimab and cilgavimab are authorized for pre-exposure prophylaxis in patients 12 years and older weighing at least 40 kg who are not currently infected with SARS-CoV-2 and have not had a known recent exposure AND:
- Who have moderate or severe immune compromise due to a medical condition or immunosuppressive medication AND may not mount an adequate immune response to COVID-19 vaccination OR
- For whom COVID-19 vaccination is not recommended dur to a history of severe adverse reaction to a COVID-19 vaccine or COVID-19 vaccine component
The combination of tixagevimab and cilgavimab is effective against the Omicron subvariants currently circulating in the US.29
References
- National Institutes of Health (NIH). COVID-19 Treatment Guidelines. Overview of COVID-19. Last updated April 29, 2022. https://www.covid19treatmentguidelines.nih.gov/overview/overview-of-covid-19/
- Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708-1720. doi:10.1056/NEJMoa2002032
- Garg S, Kim L, Whitaker M, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 — COVID-NET, 14 states, March 1-30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:458-464. doi:10.15585/mmwr.mm6915e3
- Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020;395:507-513. doi:10.1016/S0140-6736(20)30211-7
- Modified from Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020;109:102433. doi:10.1016/j.jaut.2020.102433
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72,314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239-1242. doi:10.1001/jama.2020.2648
- Phua J, Weng L, Ling L, et al. Intensive care management of coronavirus disease 2019 (COVID-19): Challenges and recommendations. Lancet Respir Med. 2020;8:506-517. doi:10.1016/S2213-2600(20)30161-2
- Ko JY, Danielson ML, Town M, et al. Risk factors for coronavirus disease 2019 (COVID-19)-associated hospitalization: COVID-19-associated hospitalization surveillance network and behavioral risk factor surveillance system. Clin Infect Dis. 2021;72:e695-e703. doi:10.1093/cid/ciaa1419
- Centers for Disease Control and Prevention (CDC) COVID-19 response team. Severe outcomes among patients with coronavirus disease 2019 (COVID-19) — United States, February 12–March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:343-346. doi:10.15585/mmwr.mm6912e2
- Centers for Disease Control and Prevention (CDC). COVID-19. People With Certain Medical Conditions. Updated May 2, 2022. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html
- Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846-848. doi:10.1007/s00134-020-05991-x
- Lippi G, Plebani M. Laboratory abnormalities in patients with COVID-19 infection. Clin Chem Lab Med. 2020;58:1131-1134. doi:10.1515/cclm-2020-0198
- Bersanelli M. Controversies about COVID-19 and anticancer treatment with immune checkpoint inhibitors. Immunotherapy. 2020;12:269-273. doi:10.2217/imt-2020-0067
- Chen L, Liu HG, Liu W, et al. Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia. Chin J Tuberc Respir Dis. 2020;43:E005 [Abstract in English]. doi:10.3760/cma.j.issn.1001-0939.2020.0005
- Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. doi:10.1001/jama.2020.1585
- Kambhampati AK, O’Halloran AC, Whitaker M, et al. COVID-19–associated hospitalizations among health care personnel — COVID-NET, 13 states, March 1-May 31, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1576-1583. doi:10.15585/mmwr.mm6943e3
- National Institutes of Health (NIH). COVID-19 Treatment Guidelines. Infection control. Last updated May 31, 2022. https://www.covid19treatmentguidelines.nih.gov/management/critical-care-for-adults/infection-control/
- Lurie N, Saville M, Hatchett R, Halton J. Developing Covid-19 vaccines at pandemic speed. N Engl J Med. 2020;382:1969-1973. doi:10.1056/NEJMp2005630
- US Food and Drug Administration (FDA). Emergency use authorization (EUA) of the Pfizer-BioNTech COVID-19 vaccine to prevent coronavirus disease 2019 (COVID-19). Revised May 17, 2022. https://www.fda.gov/media/153715/download
- US Food and Drug Administration (FDA). Emergency use authorization (EUA) of the Moderna COVID-19 vaccine to prevent coronavirus disease 2019 (COVID-19). Revised January 31, 2022. https://www.fda.gov/media/144637/download
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603-2615. doi:10.1056/NEJMoa2034577
- Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403-416. doi:10.1056/NEJMoa2035389
- Centers for Disease Control and Prevention (CDC). COVID-19 Vaccine Boosters. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/booster-shot.html
- Oliver SE, Gargano JW, Scobie H, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Janssen COVID-19 vaccine — United States, February 2021. MMWR Morb Mortal Wkly Rep. 2021;70:329-332. doi:10.15585/mmwr.mm7009e4
- Centers for Disease Control and Prevention (CDC). FDA and CDC lift recommended pause on Johnson & Johnson (Janssen) COVID-19 vaccine use following thorough safety review. Last reviewed April 23, 2021. https://www.cdc.gov/media/releases/2021/fda-cdc-lift-vaccine-use.html
- Centers for Disease Control and Prevention (CDC). Vaccines & Immunizations. COVID-19 Vaccination & Professional Resources. Last reviewed May 13, 2022. https://www.cdc.gov/vaccines/covid-19/index.html
- US Food and Drug Administration (FDA). Emergency use authorization (EUA) of REGEN-COV® (casirivimab and imdevimab). Revised January 2022. https://www.fda.gov/media/145611/download
- US Food and Drug Administration (FDA). Emergency use authorization (EUA) of bamlanivimab and etesevimab. Revised January 2022. https://www.fda.gov/media/145802/download
- US Food and Drug Administration (FDA). Emergency use authorization for EVUSHELD™ (tixagevimab co-packaged with cilgavimab). Revised May 2022. https://www.fda.gov/media/154701/download





