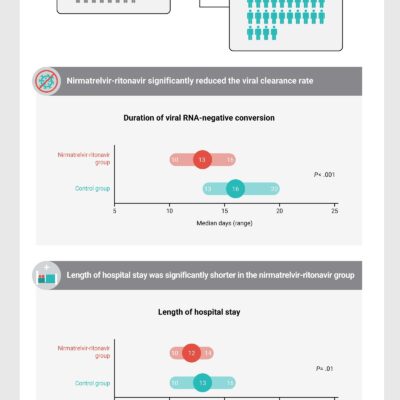
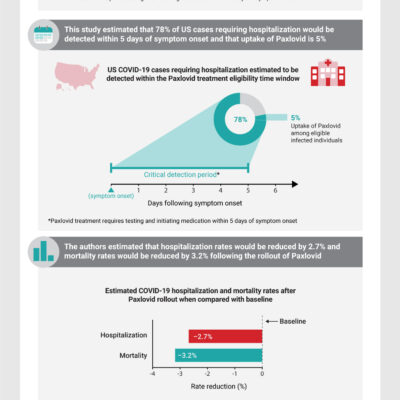
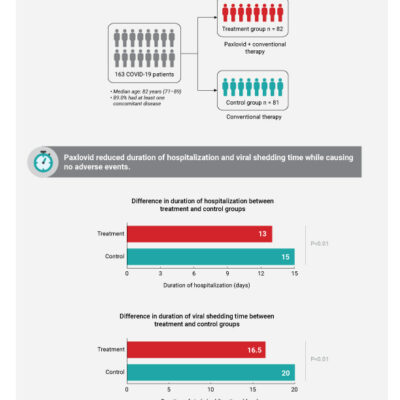
EUA Granted for Monoclonal Antibody for Hospitalized People With COVID-19
The monoclonal antibody, tocilizumab, blocks the interleukin-6 (IL-6) receptor and reduces inflammation caused by an overactive immune reaction to COVID-19. It was granted Emergency Use